Explain How Pure Substances and Mixtures Are Different
A pure substance consists only of one element or one compound. Types of compounds - definition.
Difference Between Pure Substance And Mixture Definition Composition Properties Examples
The classification of matter.
. When we have a matter first we ask the question whether it has a constant composition and properties. Gold silver water etc. Impure Substance - definition Impure materials may be mixtures of elements mixtures of compounds or mixtures of elements and compounds and has no definite composition.
A pure substance is either a compound or an element. Survey Did this page answer your question. A Mixture contains two or more.
What Is A Mixture. Pure Substance - definition A material that is composed of only one typeof particle. A Mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
Mixtures are physically combined structures that can be separated into their original components. The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. In contrast mixtures contain two or more substances so they can be separated.
A pure substance is made up of the same kind of molecules whereas mixture is made up of two. A pure substance will remain as it is no matter how fine it is divided. It has a definite composition and constant properties.
All matter can be classified as either a pure substance or a mixture. 8 rows Substances which have a specific composition and cannot be separated into any constituents are. Pure substance cannot be separated into two or more substances by any mechanical or physical method.
Uniform composition and properties of a matter indicate pure substance whereas mixtures contain variable composition and different properties. The major difference between pure substances and mixture is that pure substances have a specific composition of constituent while a. Mixtures and Pure Substances are different because a mixture is a combination of two pure substances making in impure Think about it like dog breeding.
HETEROGENOUS MIXTURES HOMOGENOUS MIXTURES All components of the mixture are visible because they do not mix together Particles not distributed evenly EX. Therefore the properties are uniform throughout the sample. 7 rows The matter is divided into two basic categories as pure substance and mixtures.
The main difference between pure substance and mixture lies in their composition. All pure substances have characteristic melting and boiling points. A mixture consists of two or more different substances not chemically joined together.
A pure substance consists of only one kind of matter that is all the particles are same. A pure substance contains only one kind of compound. However its important to look at them individually in order to understand the nature of these substances better.
A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions suspensions and colloids. Mixtures are also called impure substances because it is composed of different kinds of components. What is the difference between Pure Substance and Mixture.
A pure substance usually participates in a chemical reaction to form predictable products. A pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. The pure substances possess similar properties and composition throughout on the other hand in mixtures properties and composition vary as the constituents are mixed in indefinite proportion.
1 a pure substance has a definite chemical formula. Sand and water vegetable soup oil and water Homogeneous mixtures Components cannot be distinguished from each other appear as one substance Particles distributed evenly throughout EX. Mixtures are composed of several kinds of compounds.
The physical and chemical. Pure substances are homogenous. Pure substances A pure substance has a definite and constant composition like salt or sugar.
Mixtures can either be heterogeneous and homogeneous. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary. Pure substances also have fixed shapes and structures.
It can be the same molecule or atom. Matter can be broken down into two categories. Pure substances are composed of a single element or compound while homogeneous mixtures are composed of multiple different elements or compounds.
The components of a mixture can. A pure-bred dog is like a pure substance while a mixed-breed could be considered a mixture Still stuck. Get 1-on-1 help from an expert tutor now.
It cannot be split into simpler substances by physical means. Pure substances are defined as substances made up of only one kind of atom or molecule. Pure substances and mixtures.
A mixture is composed of two or more substances that are combined mechanically as opposed to chemically. If it is pure then the next question is if the substance can be broken down into any other substance by a chemical method. A pure substance is a form of matter that has a definite constant composition and distinctive properties.
2 a mixture has two or more kinds of matter and particles. Chemical bonds between the components are neither broken nor formed. Pure substances are further classified into elements and compounds.
A Pure Substance is matter which cannot be separated into its basic components by using a physical or a chemical process. Pure substances are further broken down into elements and compounds. On the other hand a mixture is a combination of two or more substances in which they retain their properties.
A chemical substance is composed of one type of atom or molecule.
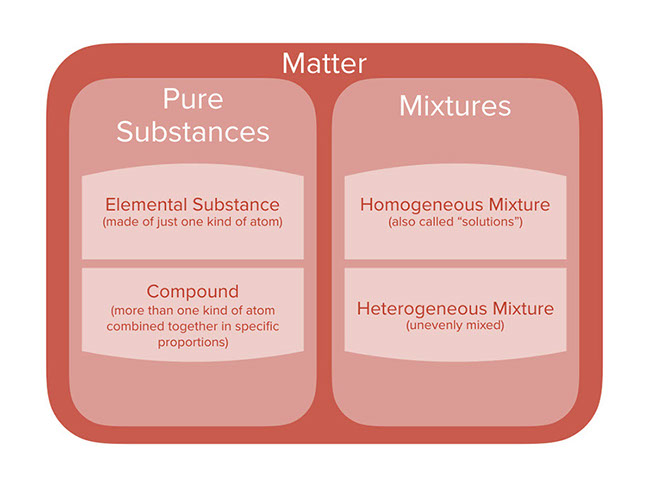
Lesson Categories Of Chemicals And Mixtures
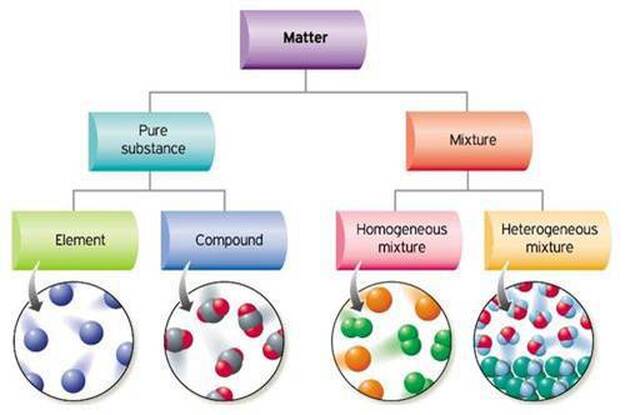
Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department

What Are The Types Of Pure Substances And Mixtures A Plus Topper
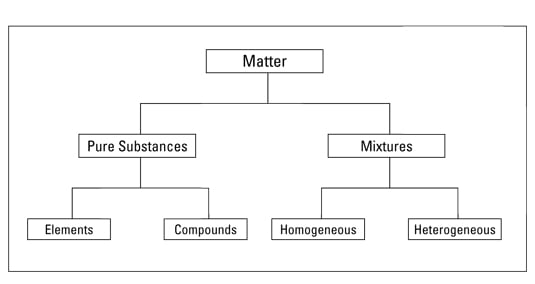
No comments for "Explain How Pure Substances and Mixtures Are Different"
Post a Comment